Why Are 1 Chlorobutane And 2 Chlorobutane Structural Isomers
Why Are 1 Chlorobutane And 2 Chlorobutane Structural Isomers. Those are the only structural isomers. Add your answer and earn points.
If you're aware of your "Why" and decide to follow it with determination and determination, you can comprehend everything that happens on your way because you look at it through the perspective that is "Why". When you've identified you "Why", you will be able find it in your "Way". How do those things differ? "Why" is your purpose. "Way" is your path. When you realize you're "Why", your path will naturally have a reason. It makes life more significant and complete as you understand why you're on your journey in first place.
Do you find that"The "Why" always comes first? Do you know how to get there and then figure out an "Why"? You might be asking yourself. What's the most important thing to do? The good news is that both could come first. If"why" is first "Why" comes before the way, your capability to make use of the power of significance can be achieved more quickly and also be more effective.
Imagine it this way. Have you ever wondered how people enjoy so much packing to go on vacation? They put in weeks of the anticipation of their trip, looking forward to those sunny days on beaches in the tropical sun or trips down the slopes at their favorite ski resort. So , they choose each piece of luggage that is placed in the luggage with diligence.
As you prepare to embark on an exciting trip, you'll be directed towards the main goal of the excursion. It's the reason it's easier to pack for a trip than it is in the aftermath to empty your bags. The same principle applies to our daily lives. Whatever your path, you'll be able to do things greater because you understand the motivation behind why you're there.
Which is more flammable, hexane or potassium sulfate? Solve any question of organic. Those are the only structural isomers.
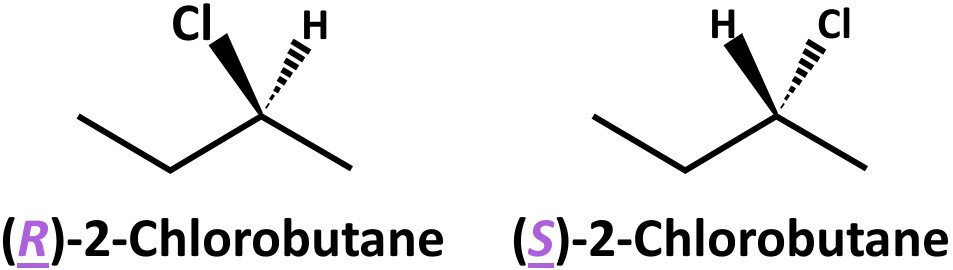
As Sven Has Said, There Are Four Structural Isomers One Of Which Is Chiral And Exists As A Pair Of Enantiomers.
Hence, option b is correct. There are only two structural isomers of c3h7br. Add your answer and earn points.
Those Are The Only Structural Isomers.
What changes in color occur when bromine. There exist 4 isomers in chlorobutane. Which is more flammable, hexane or potassium sulfate?.
You Can Request For Your Textbook To Be Answered.
1 see answer advertisement advertisement andrewn6581 is waiting for your help. 3 iterations of this structure are bonded together by single bonds from the bottom right c h 2 of one structure to the bottom left c h 2 of the next. Solve any question of organic.
The Stereoisomerism In Which The Isomers Have Different Spatial Arrangements Of Groups/Atoms Around A Chiral Atom Is Called Optical Isomerism.
Isomers are compounds that include the same number of atoms, i.e., they have the exact empirical formula, but vary from. Would you expect hexane to be soluble in water? There are four structural isomers with the molecular formula c4h9cl.
Which Is More Flammable, Hexane Or Potassium Sulfate?
If you push the attached group further.it would only give you the same sturcture from. They both have a methyl substituent, just positioned at different spots. The structures are shown below.
Post a Comment for "Why Are 1 Chlorobutane And 2 Chlorobutane Structural Isomers"